Publication – Copper(I)-catalyzed azide–alkyne cycloadditions in microflow: Catalyst activity, high-T operation, and an integrated continuous copper scavenging unit
ChemSusChem. doi: 10.1002/cssc.201200323
- Alvaro Carlos Varas, Dr. Timothy Noël, Dr. Qi Wang, Prof. Dr. Volker Hessel*
- Eindhoven University of Technology, Micro Flow Chemistry & Process Technology, Den Dolech 2, Helix, STW 2.49, P.O. Box 513, 5600 MB Eindhoven, The Netherlands
The Asia FLLEX module was used as a copper scavenging/extracting unit.
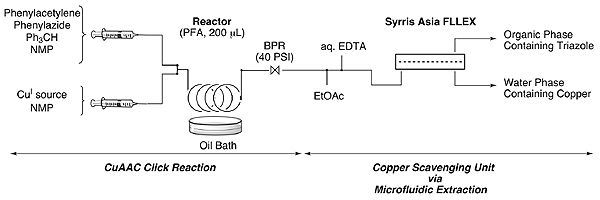
Abstract: A continuous-flow synthesis for the CuI-catalyzed azide–alkyne cycloaddition reaction using [Cu(phenanthroline)(PPh3)2]NO3 as a homogeneous catalyst is developed (up to 92 % isolated yield). Elevated temperatures allow achieving full conversions and using lower catalyst loadings. Residual copper in the triazole compound is efficiently removed via an inline extraction process, employing aqueous EDTA as a copper scavenger.
An Asia system and the Asia FLLEX module was used as a copper scavenging/extracting unit for this paper.

