Publication – Expedient preparation of nazlinine and a small library of indole alkaloids using flow electrochemistry
Organic Letters. 2014, 16, 4618-4621
Mikhail A. Kabeshov, Biagia Musio, Philip R. D. Murray, Duncan L. Browne, Steven V. Ley
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK
This paper describes the use Syrris’s Asia Flux module to achieve full natural product synthesis of Nazlinine. The building blocks of Nazlinine, along with a library of unnatural ?-methoxyamine congeners, were generated by Flow Electrochemistry very rapidly and in very high yields. These key ?-methoxyamine oxidation products were telescoped into a microwave mediated Pictet-Spengler reaction to facilitate a two-step, high yielding synthesis of Nazlinine. The Electrochemically inspired intermediate scaffold could easily lead to the preparation of further unnatural relatives of Nazlinine such as Komaroidine or Schobericine.
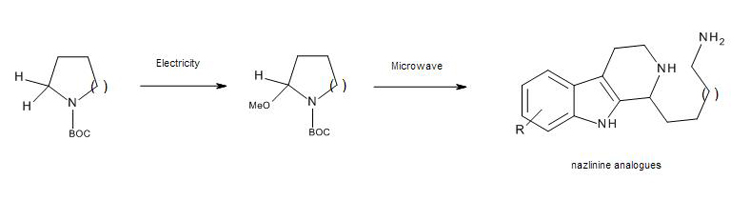
Abstract: An expedient synthesis of the indole alkaloid nazlinine is reported. Judicious choice of flow electrochemistry as an enabling technology has permitted the rapid generation of a small library of unnatural relatives of this biologically active molecule. Furthermore, by conducting the key electrochemical Shono oxidation in a flow cell, the loading of the electrolyte can be significantly reduced to 20 mol % while maintaining a stable, broadly applicable process.
This paper uses the Asia Flux. Learn more about the product this chemistry was performed on:
For more information, contact us.

